A limestone draw kiln, used in the early 1900s, was a type of vertical kiln designed to produce quicklime (calcium oxide) by heating limestone (calcium carbonate) in a process called calcination. Here's how it worked:
Structure of the Kiln:
Vertical Shaft**: The kiln was typically a tall, cylindrical or rectangular structure, large exterior stones of kiln and lined with fire/ refractory bricks to withstand high temperatures. It had a vertical shaft where the limestone is loaded.
.The fire chambers are at the base of the kiln. There are 2 fire chambers, directly opposite these openings allow air to flow to generate
combustion.
_edited_edited_edited_ed.jpg)
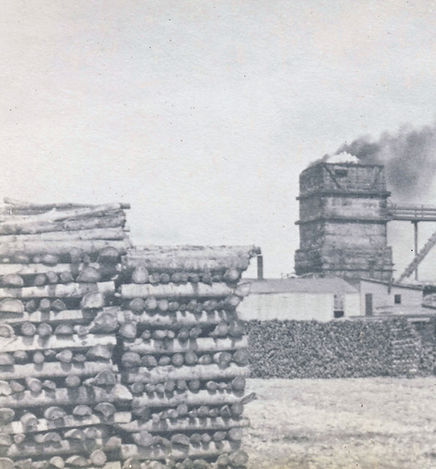
Fuel - The fire chambers of the kiln are loaded with wood.The firewood can from farmers that were clearing trees from their land.
The fuel provides the heat needed for the calcination process.Ignition: The fuel was ignited at the base of the kiln, and the heat absorbed by the inner lining of refractory bricks.
The temperature inside the kiln reached around 900–1000°C (1650–1830°F), which is necessary for calcination.
Once the limestone is calcined,, the quicklime exits via gravity thru the draw chute at the bottom of the kiln.
The quicklime is loaded onto railcars for delivery to customers in Canada
Exhaust : The carbon dioxide gas and other combustion byproducts exited the kiln through the top of the kiln. Water that is in the limestone is converted to steam that you can see exiting.
Calcination: As the heat moved upward, the limestone (CaCO₃) decomposed into quicklime (CaO) and carbon dioxide (CO₂) gas. The chemical reaction is:
CaCO3→CaO+CO2CaCO3→CaO+CO2